
Cardiac Amyloidosis Uncovered
- The first FDA cleared AI based screening tool, to help detect amyloidosis from an echocardiogram

EchoGo® Amyloidosis
Designated as a FDA Device Breakthrough, this pioneering clinical AI is designed to assist in the detection of Cardiac Amyloidosis during routine cardiovascular assessments. It analyzes echocardiogram data and provides critical insights to support physicians in detecting this often underdiagnosed disease.
EchoGo® Amyloidosis operates in tandem with EchoGo® Heart Failure to identify complex subtypes and underlying causes of heart failure. Together, these tools enhance diagnostic accuracy, enable systematic screening, and support timely access to potentially life-saving therapies.

Proven to Detect Amyloidosis with Precision
Ultromics Echo-based diagnostic solutions now support the detection and management of amyloidosis, utilizing AI to consolidate data, generate insights, and prioritize patient triage. By streamlining communication, these tools help ensure patients with amyloidosis receive timely follow-up, improving outcomes and supporting more effective, continuous care.
<BR>
Improving diagnosis and outcomes is critical
Early and accurate detection of cardiac amyloidosis is critical due to its progressive and irreversible nature, where delayed diagnosis can lead to advanced heart failure and significantly impact quality of life and survival rates[1,2]. Cardiac Amyloidosis is often mistaken for alternative diseases including HFpEF meaning cases are often missed. Timely identification allows for the initiation of specific treatments that can slow disease progression, such as stabilizers and gene-silencing therapies for ATTR or chemotherapy for AL Amyloidosis.
Echocardiography is a powerful tool for evaluating cardiac structure & function and is central to the early detection and monitoring of disease. However, there are some diseases that are very challenging for even the most expert clinician to detect on an echocardiogram.
5+ MD visits before diagnosis (ATTR)3
14% of HF population with Amyloid4
65% 5-year mortality (AL)5
52% 90-day readmission6
Data-Driven Innovation
“Novel AI-based diagnostic tools such as EchoGo® Amyloidosis from Ultromics should help facilitate disease identification, particularly in clinics and hospitals restricted by expertise and resource. ”
Research and Partnerships


Tested and Proven By Leading Cardioloists
Seamless Integration with EchoGo® Platform
The EchoGo® platform operates seamlessly in the background, running Ultromics’ advanced algorithms at scale. Designed to integrate effortlessly into your workflow, EchoGo® connects with your existing IT infrastructure and provides seamless access to reports.
Integrate Seamlessly
- Easily implemented, with no hardware/software at site.
- Integrates with existing workflows and IT stacks.
- Secure cloud VPN connection and data anonymized.
Analyze Data
- EchoGo® analyzes data, identifies suspected findings, and generates a report.
- A report is sent to PACS for the interpreting physician.
- Operates in an automated DICOM workflow.
Get Reimbursed*
- Inpatient: NTAP Code ICD-10 XXE2X19 – $1,000 per analysis (Medicare-covered).
- Outpatient: HCPCS Code C9786, Clinical APC Code 574 – $285 per analysis (covered by Medicare and select commercial insurers).
-
EchoGo® Amyloidosis operates in tandem with EchoGo® Heart Failure which is eligible for reimbursement.*
Scale with Ease
- Deploy Ultromics’ AI algorithms across any care setting—from hospitals to clinics—without workflow disruption.
- Minimal training to onboard new staff with ease.
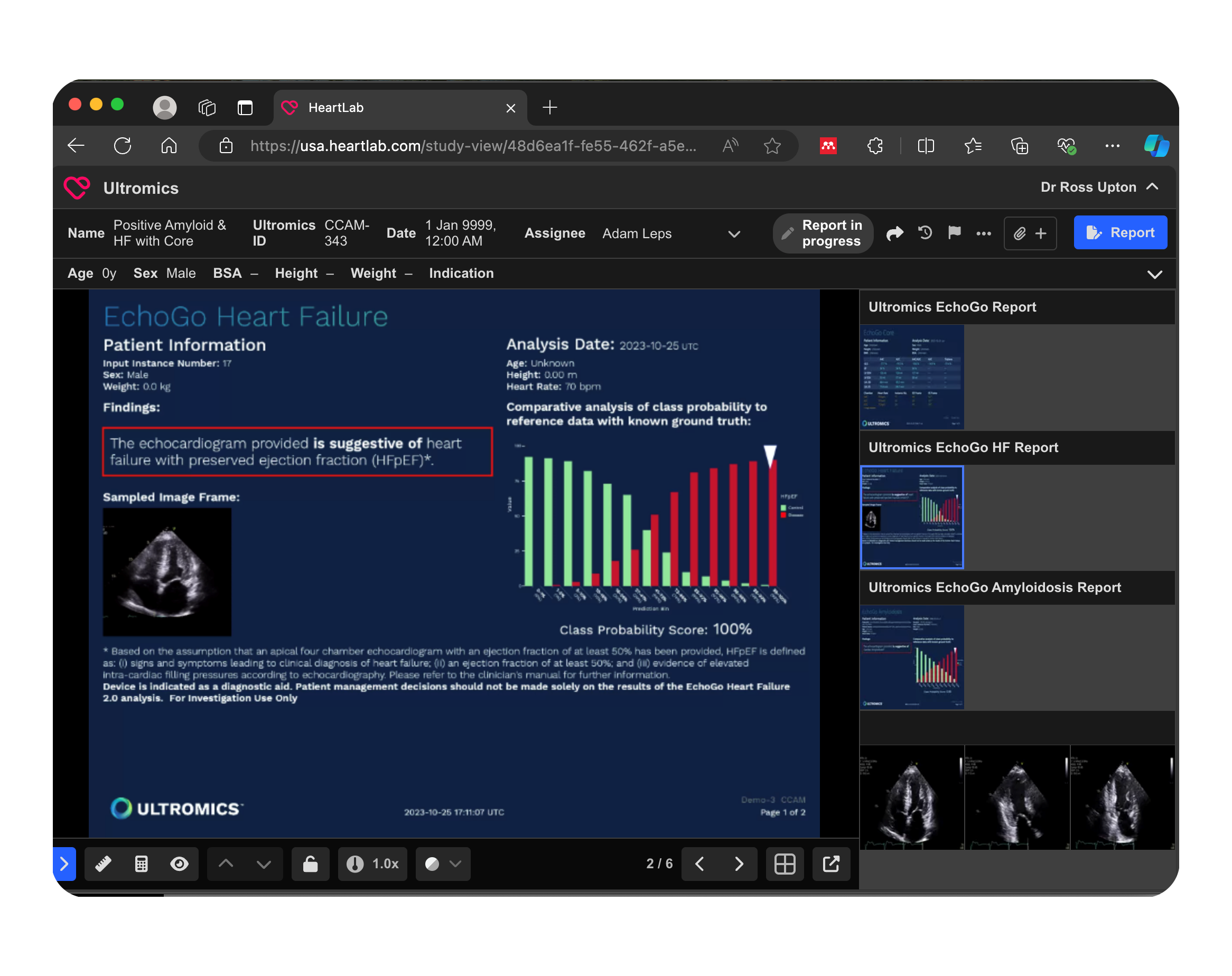
AI-Driven Cardiology Diagnostics
This integrated PACS solution leverages AI-powered analysis to suggest heart failure classifications directly from echocardiogram data. The automated reporting within PACS allows clinicians to view diagnostic insights directly in the workflow. This streamlined approach supports faster, data-driven clinical decisions.
QC + secured
-
CyberEssentials, ISO 27001, and ISO 13485
-
HIPAA Compliant
Vendor Neutral
-
Standard interfaces into all PACS
-
Secure, encrypted VPN
Cloud Native
-
Optimal resource savings, with no new hardware or software required
-
No need to learn or train on additional systems
Dashboard Analytics
-
CyberEssentials, ISO 27001, and ISO 13485
-
HIPAA Compliant
Secure by design
EchoGo® is a secure SaaS model. It complies with all relevant privacy and security regulations, including HIPPA and SOC 2 Type 2. Data is anonymized, encrypted in transit and at rest, ensuring patient confidentiality at all times.








Latest Research
In partnership with Amyloidosis specialists at Mayo Clinic, our research investigates the diagnostic performance of EchoGo® Amyloidosis in a clinical setting. Using a robust testing data set of 3,000 cases, EchoGo® demonstrated a high diagnostic accuracy:
0.93 AUC
84% Sensitivity
92% Specificity

References:
- Gillmore JD, Damy T, Fontana M, Hutchinson M, Lachmann HJ, Martinez-Naharro A, et al. A new staging system for cardiac transthyretin amyloidosis. European heart journal. 2018 Aug;39(30):2799–806.
- Kumar S, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, Colby C, et al. Revised prognostic staging system for light chain amyloidosis incorporating cardiac biomarkers and serum free light chain measurements. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012 Mar;30(9):989–95.
- Gertz M, Adams D, Ando Y, Beirão JM, Bokhari S, Coelho T, et al. Avoiding misdiagnosis: expert consensus recommendations for the suspicion and diagnosis of transthyretin amyloidosis for the general practitioner. BMC Family Practice. 2020 Sep 23;21(1).
- Hahn VS, Yanek LR, Vaishnav J, Ying W, Vaidya D, Lee YZJ, et al. Endomyocardial Biopsy Characterization of Heart Failure With Preserved Ejection Fraction and Prevalence of Cardiac Amyloidosis. JACC Heart failure. 2020 Sep 1;8(9):712–24.
- Escher F, Senoner M, Doerler J, Zaruba MM, Messner M, Mussner-Seeber C, et al. When and how do patients with cardiac amyloidosis die? Clinical Research in Cardiology. 2019 May 27;109(1):78–88.
- Berthelot E, Amaury Broussier, Hittinger L, Donadio C, Rovani X, Salengro E, et al. Patients with cardiac amyloidosis are at a greater risk of mortality and hospital readmission after acute heart failure. ESC heart failure. 2023 Apr 13;10(3):2042–50.
Discover the full potential of EchoGo®
Access both HFpEF and Amyloidosis in one seamless platform.


