
Clinical Utility Of Screening For Transthyretin Cardiac Amyloidosis In HFpEF
- | By Ultromics
Will Hawkes1, Jeremy Slivnick2, Ashley Akerman1, Jorge Oliveira1, Alberto Gomez1, Gary Woodward1, Izhan Hamza3, Viral K. Desai3, Zachary Barrett-O’Keefe3, Martha Grogan3, Christopher G. Scott3, Halley N. Davison3, Juan Cotella2, Matthew Maurer4, Stephen Helmke4, Marielle Scherrer-Crosbie5, Marwa Soltani5, Akash Goyal6, Karolina M. Zareba6, Richard Cheng7, James N. Kirkpatrick7, Tetsuji Kitano8, Masaaki Takeuchi8, Viviane Tiemi Hotta9, Marcelo Luiz Campos Vieira9, Pablo Elissamburu10, Ricardo E. Ronderos10, Aldo Prado11, Efstratios Koutroumpakis12, Anita Deswal12, Amit Pursnani13, Nitasha Sarswat1, Amit M. Patel, MD14, Karima Addetia1, Frederick L. Ruberg15, Michael Randazzo2, Federico M. Asch16, Jamie O’Driscoll17, Nora Al-Roub18, Jordan B. Strom18, Liam Kidd19, Sarah Cuddy19, Ross Upton1, Roberto M. Lang2, Patricia A. Pellikka3
1University of Chicago, Chicago, IL; 2Ultromics, Ltd., Oxford, UK; 3Mayo Clinic, Rochester, MN; 4Columbia University, New York, NY; 5University of Pennsylvania, Philadelphia, PA; 6Ohio State University, Columbus, OH; 7University of Washington, Seattle, WA; 8Hospital of University of Occupational and Environmental Health, Kitakyushu, Japan;9Heart Institute (InCor), Sao Paolo, Brazil; 10ICBA, Buenos Aires, Argentina; 11Centro Privado de Cardiología, Tucuman, Argentina; 12University of Texas MD Anderson Cancer Center, Houston, Texas; 13NorthShore, Evanston, IL; 14University of Virginia Medical Center, Charlottesville, VA; 15Boston University Chobanian & Avedisian School of Medicine, Boston, MA; 16MedStar Health Research Institute, Washington, DC; 17Canterbury Christ Church University; 18Beth Israel Deaconess Medical Centre; 19Brigham & Women’s Hospital
Background
Screening algorithms for cardiac amyloidosis (CA), including artificial intelligence (AI), show promise for the discrimination and classification of disease. However, evaluation of clinical utility is necessary to evaluate the benefits and harms associated with the use of screening algorithms across the spectrum of patient presentations.
Methods
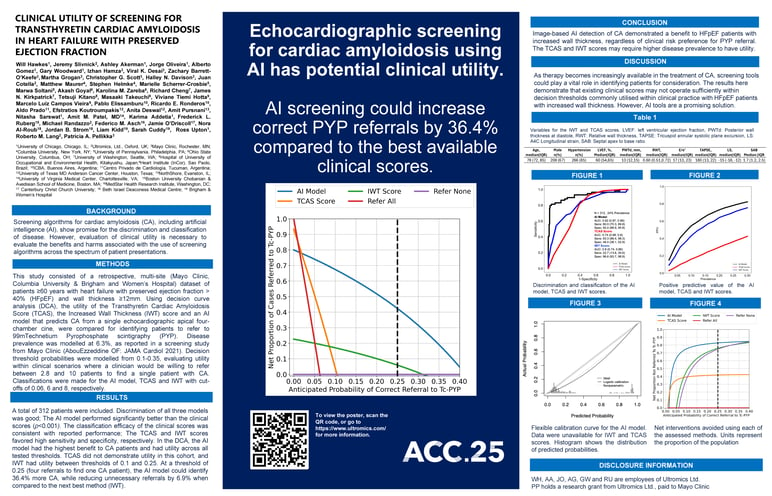
This study consisted of a retrospective, multi-site (Mayo Clinic, Columbia University & Brigham and Women’s Hospital) dataset of patients ≥60 years with heart failure with preserved ejection fraction > 40% (HFpEF) and wall thickness ≥12mm. Using decision curve analysis (DCA), the utility of the Transthyretin Cardiac Amyloidosis Score (TCAS), the Increased Wall Thickness (IWT) score and an AI model that predicts CA from a single echocardiographic apical four-chamber cine, were compared for identifying patients to refer to 99mTechnetium Pyrophosphate scintigraphy (PYP). Disease prevalence was modelled at 6.3%, as reported in a screening study from Mayo Clinic (AbouEzzeddine OF: JAMA Cardiol 2021). Decision threshold probabilities were modelled from 0.1-0.35, evaluating utility within clinical scenarios where a clinician would be willing to refer between 2.8 and 10 patients to find a single patient with CA. Classifications were made for the AI model, TCAS and IWT with cut-offs of 0.06, 6 and 8, respectively.
Results
A total of 312 patients were included. Discrimination of all three models was good; The AI model performed significantly better than the clinical scores (p<0.001). The classification efficacy of the clinical scores was consistent with reported performance; The TCAS and IWT scores favored high sensitivity and specificity, respectively. In the DCA, the AI model had the highest benefit to CA patients and had utility across all tested thresholds. TCAS did not demonstrate utility in this cohort, and IWT had utility between thresholds of 0.1 and 0.25. At a threshold of 0.25 (four referrals to find one CA patient), the AI model could identify 36.4% more CA, while reducing unnecessary referrals by 6.9% when compared to the next best method (IWT).




Discussion
As therapy becomes increasingly available in the treatment of CA, screening tools could play a vital role in identifying patients for consideration. The results here demonstrate that existing clinical scores may not operate sufficiently within decision thresholds commonly utilised within clinical practice with HFpEF patients with increased wall thickness. However, AI tools are a promising solution.
Conclusion
Image-based AI detection of CA demonstrated a benefit to HFpEF patients with increased wall thickness, regardless of clinical risk preference for PYP referral. The TCAS and IWT scores may require higher disease prevalence to have utility.